Sector(s)
Team Members
The Medical Device Single Audit Program (MDSAP) allows for a single Quality Management System (QMS) audit of a medical device manufacturer’s facilities, the results of which are recognized by multiple regulatory authorities.
Launched as a pilot program, MDSAP became fully operational in March 2016 and has seen significant growth since. Today, it includes approximately 7,000 certified medical device manufacturers worldwide, streamlining compliance across jurisdictions and simplifying regulatory processes.
About the project
The project followed a collaborative, user-centred approach, starting with discovery and stakeholder engagement to understand the needs of regulators, auditors, and manufacturers. From there, a comprehensive design and development plan was executed to address both functional and visual challenges.
Back to topBranding design
The project began with the creation of a professional and consistent brand identity. This included the design of a new logo, the development of brand guidelines, and the production of document templates and presentation slide decks to ensure a unified look and feel across all materials.

Information Architecture
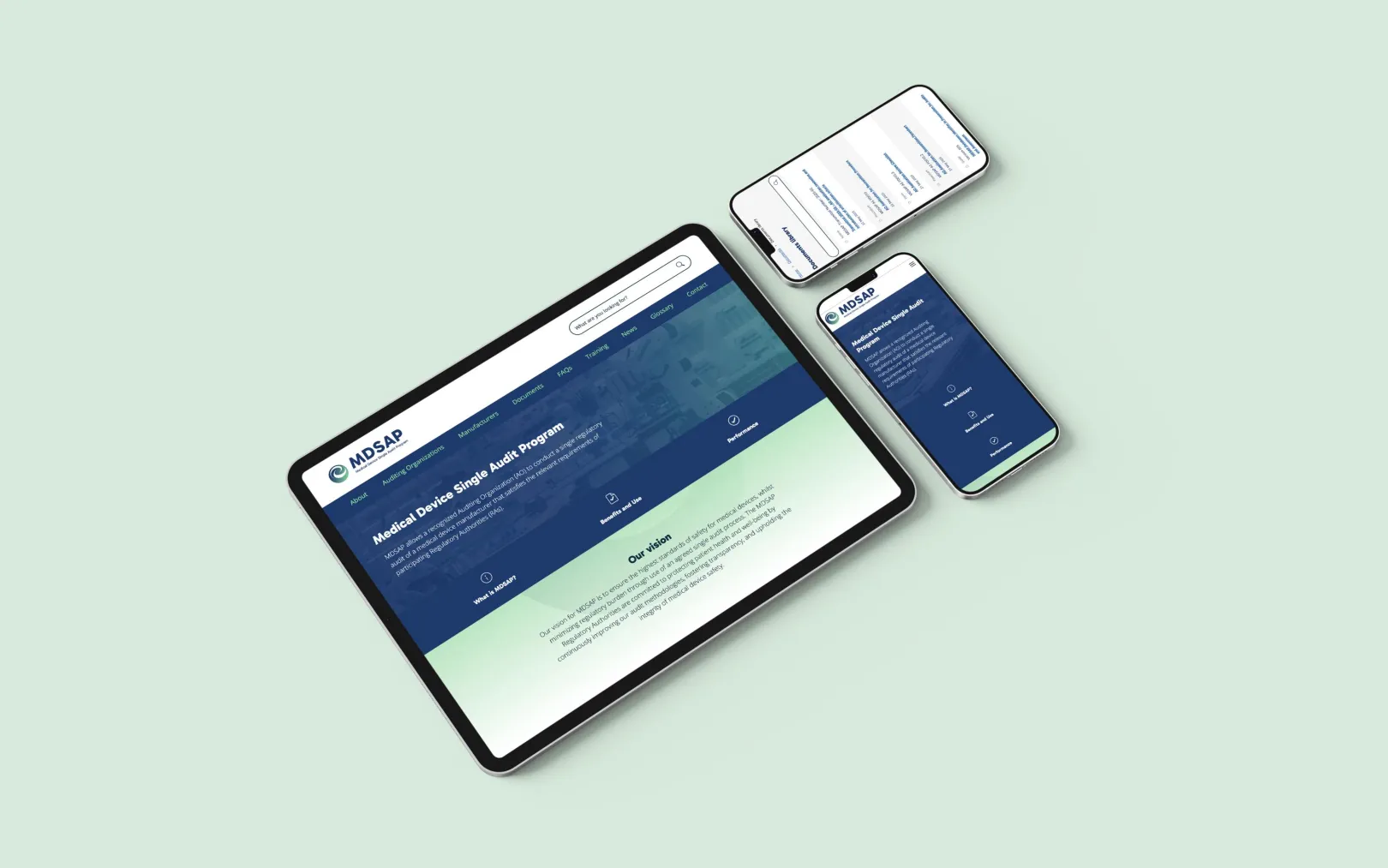
A new website was designed and developed with user experience at its core. The information architecture (IA) was carefully planned to make navigation intuitive and logical, helping users easily locate relevant content.

Site design
The design system was built around flexible, reusable components, enabling the creation of consistent page layouts that could scale with the program’s evolving content needs. This modular approach ensures that new pages and sections can be added efficiently while maintaining visual and functional consistency across the site.

Document library
A key feature of the new site is a searchable document library. Users can now browse or filter documents by category, process, or document type, removing the need to know specific document codes and vastly improving accessibility. Once viewing a document, the user can see all related documents in the process.

The outcome
The new MDSAP website and brand have significantly enhanced the program’s credibility, usability, and visibility. With improved user experience, users can now easily find audit-related materials without prior knowledge of document codes. A strong, professional brand now runs across all MDSAP communications and materials, reinforcing trust and recognition. The custom-built document library allows users to search and filter with ease, greatly improving efficiency for regulators, auditors and manufacturers.
Back to topWhy Drupal was chosen
As the Medical Device Single Audit Program (MDSAP) transitioned from a pilot to a fully operational global initiative, the need for a modern, user-friendly digital presence became clear. The project aimed to establish a strong visual identity, improve access to documentation, and deliver a seamless web experience for regulators, auditors, and manufacturers worldwide.
Technical Specifications
Drupal version:
Key modules/theme/distribution used: